INTERACT3 Review - Care Bundle with Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial
Published:
Clinical question
In patients with spontaneous intracerebral hemorrhage (ICH), does a bundle of intensive blood pressure lowering (target systolic BP <140 mm Hg within 1 hour), protocolized glucose and temperature control as well as reversal of anticoagulation reduce the risk of death or severe disability as compared to non protocolized standard of care?
This article will mainly focus on the blood pressure lowering aspect of the trial.
Pathophysiological considerations
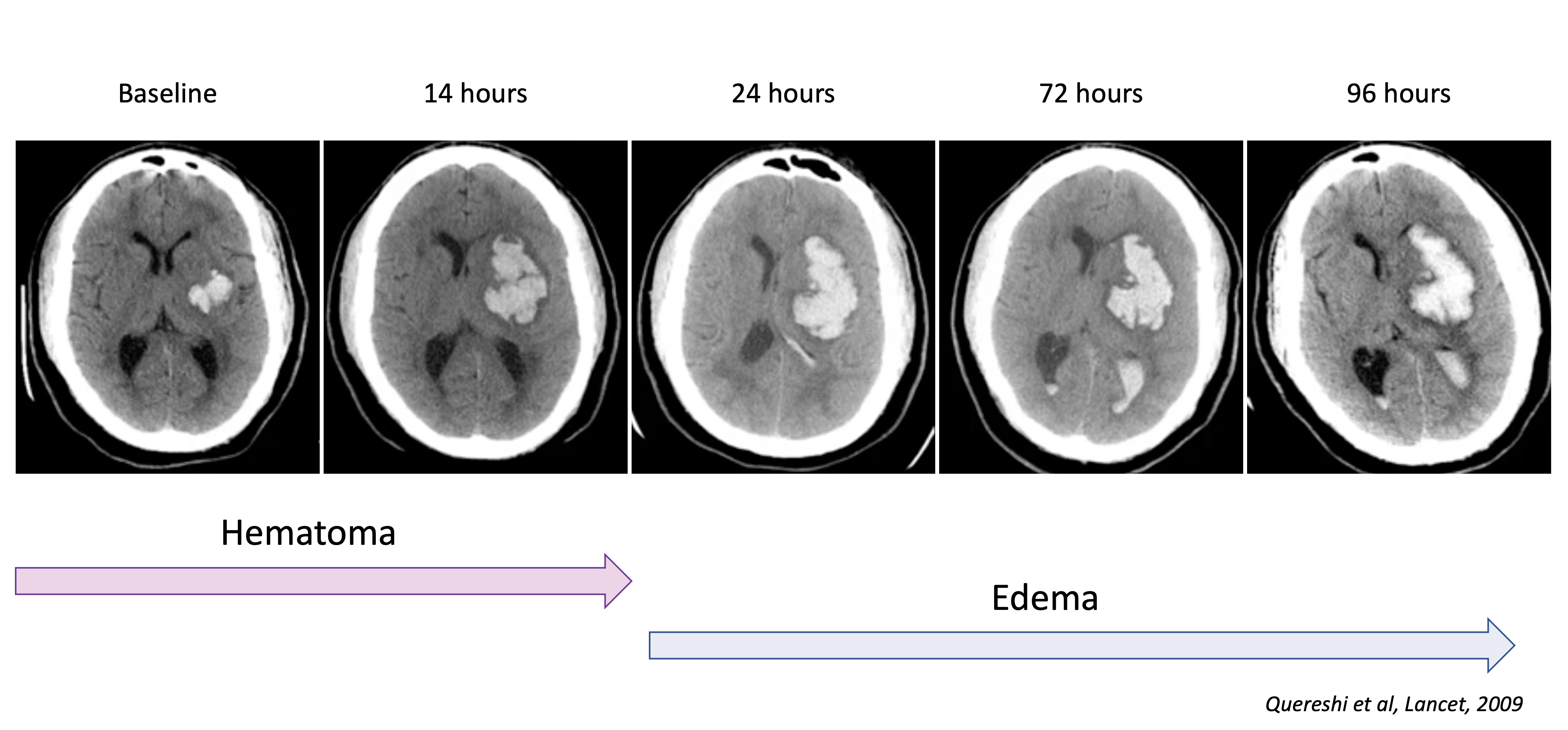
The early hours after ICH are characterized by balance act between hematoma expansion and maintaining cerebral perfusion.
Hematoma expansion
- Hematoma expansion is a major determinant of outcome in ICH
- Most hematoma expansion occurs within 3 hours of onset
- Hypertension is a major risk factor for hematoma expansion
Acute hypertensive response
- Hypertension is frequently observed in the acute phase of ICH
- Hypertension could be a autoregulatory response to maintain cerebral perfusion
- Peri-hematoma penumbra seems to be a myth, as perihematomal tissue as proportionally reduced metabolism and perfusion
The later stages seem to be driven by secondary injury, such as edema (both focal and global), inflammation and oxidative stress.
Prior evidence
RCTs
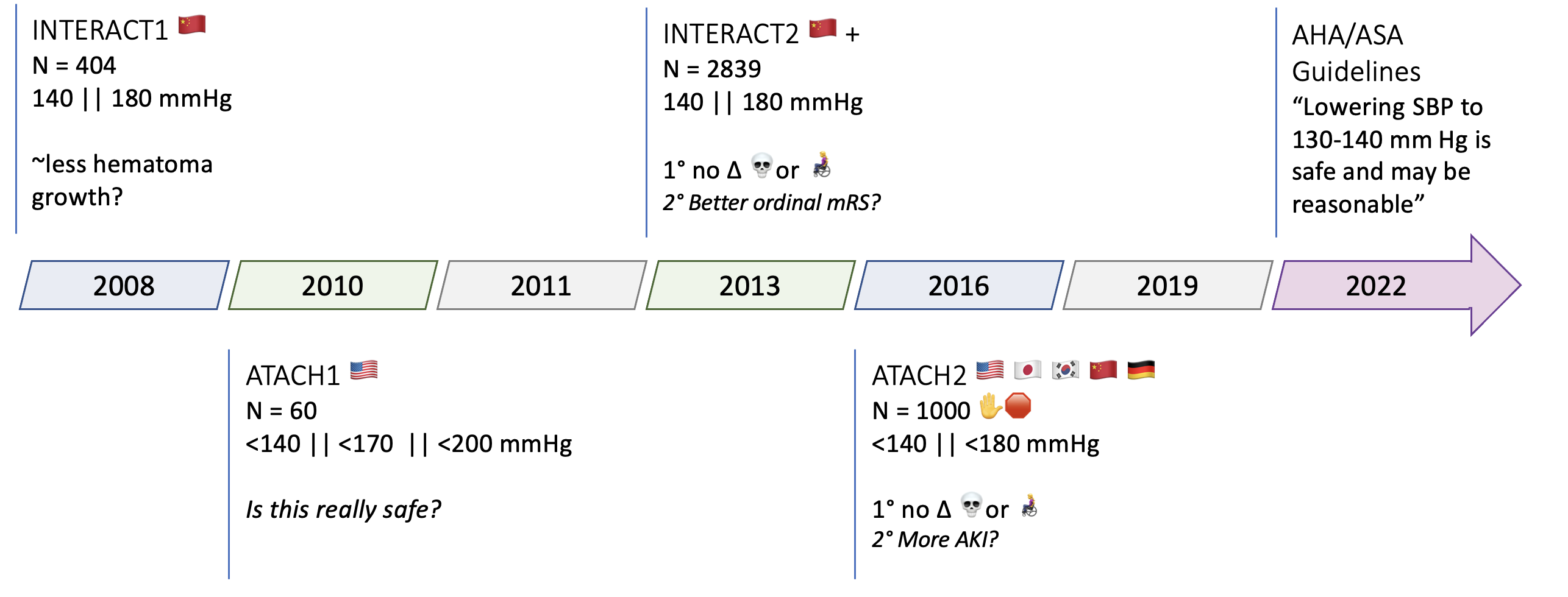
- INTERACT1 2008 – n=404, <140 vs <180mmHg, BP goals barely met, possibly less hematoma growth
- ATACH1 2010 – n=60 IV anti-hypertensive, BP goals achieved
- INTERACT2 2013 – n=2839, BP goals barely met, no diff in hematoma expansion, no diff in primary (mrs >3), tendancy toward better mrs
- ATACH2 2016 – n=1000 (stopped for futility), BP goals achieved, trend toward reduced hematoma expansion, no diff in outcome, more AKI
- AHA/ASA guidelines 2022: lowering systolic BP (SBP) to a target range of 130 to 140 mmHg is safe and may be reasonable
Bundle trials
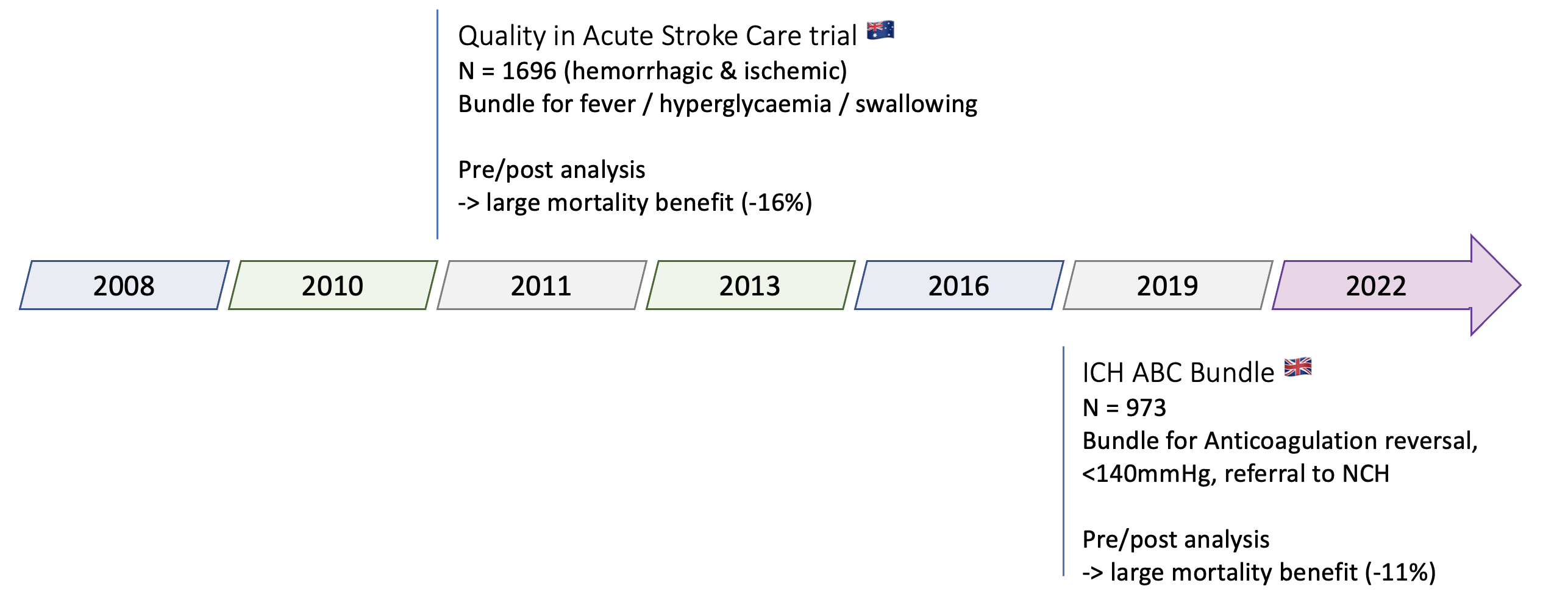
- Quality in Acute Stroke Care trial 2011 – n=1696 (hemorrhagic & ischemic), pre/post analysis, large mortality benefit (-16%)
- Bundle: management of fever / hyperglycemia / swallowing
- ICH ABC Bundle 2019 – n= 973, pre/post analysis, UK, large mortality benefit (-11%)
- Bundle: Anticoagulation reversal, <140mmHg, referral to NCH
Main critiques of prior trials:
- Achieved BP goals differed between trials (INTERACT2: 140mmHg, ATACH2: 110mmHg)
- Outcome should be assessed at 6 months, not 3 months (as traditionally done in stroke trials)
Gist: Meta-analyses found an overall benefit (shift in ordinal mRS scale) of intensive BP lowering in ICH and guidelines started mentioning / recommending a target of 130-140mmHg.
INTERACT3
Population
Site inclusion criteria
- No existing protocol for BP lowering
- Local champion
Patient inclusion criteria
- > 18y
- Spontaneous ICH, confirmed by CT
- ≤ 6 hours from onset
Patient exclusion criteria
- Evidence that ICH is secondary to AVM / aneurysm / trauma / ichemic stroke / lysis
- High likelihood that patient will not adhere to treatment / follow-up
Screening: continuous of all arriving patients (controlled by trial staff)
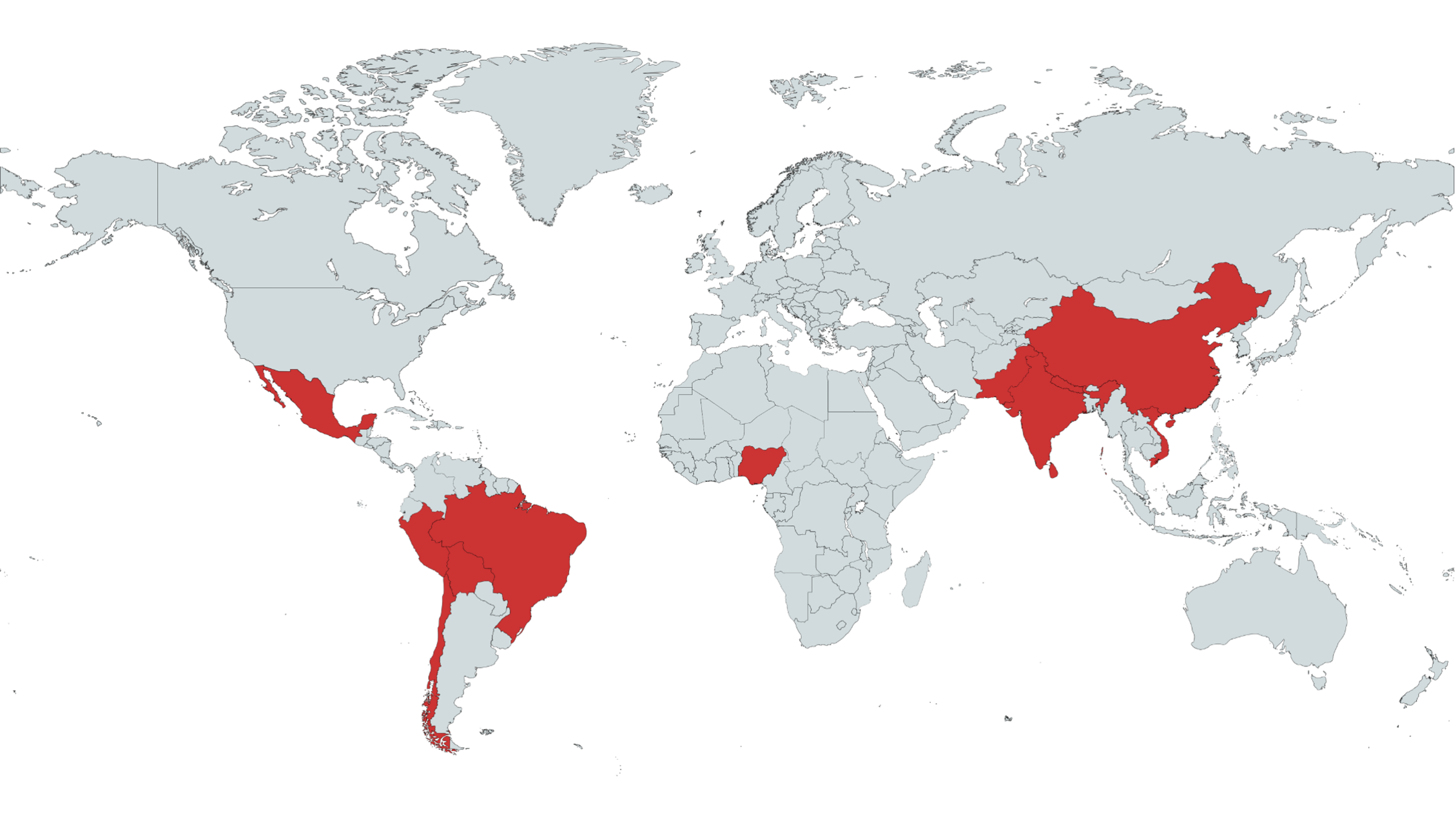
Participating countries: Brazil, China, India, Mexico, Nigeria, Pakistan, Peru, Sri Lanka, Viet Nam, Chile
Why?
- Probably no equipoise in high-income countries or existing protocols
- High & rising prevalence of hypertension and ICH in LMICs
- Trial powered by the George Institute, realising large studies, targeting global health issues (with an established trial network)
Trial design
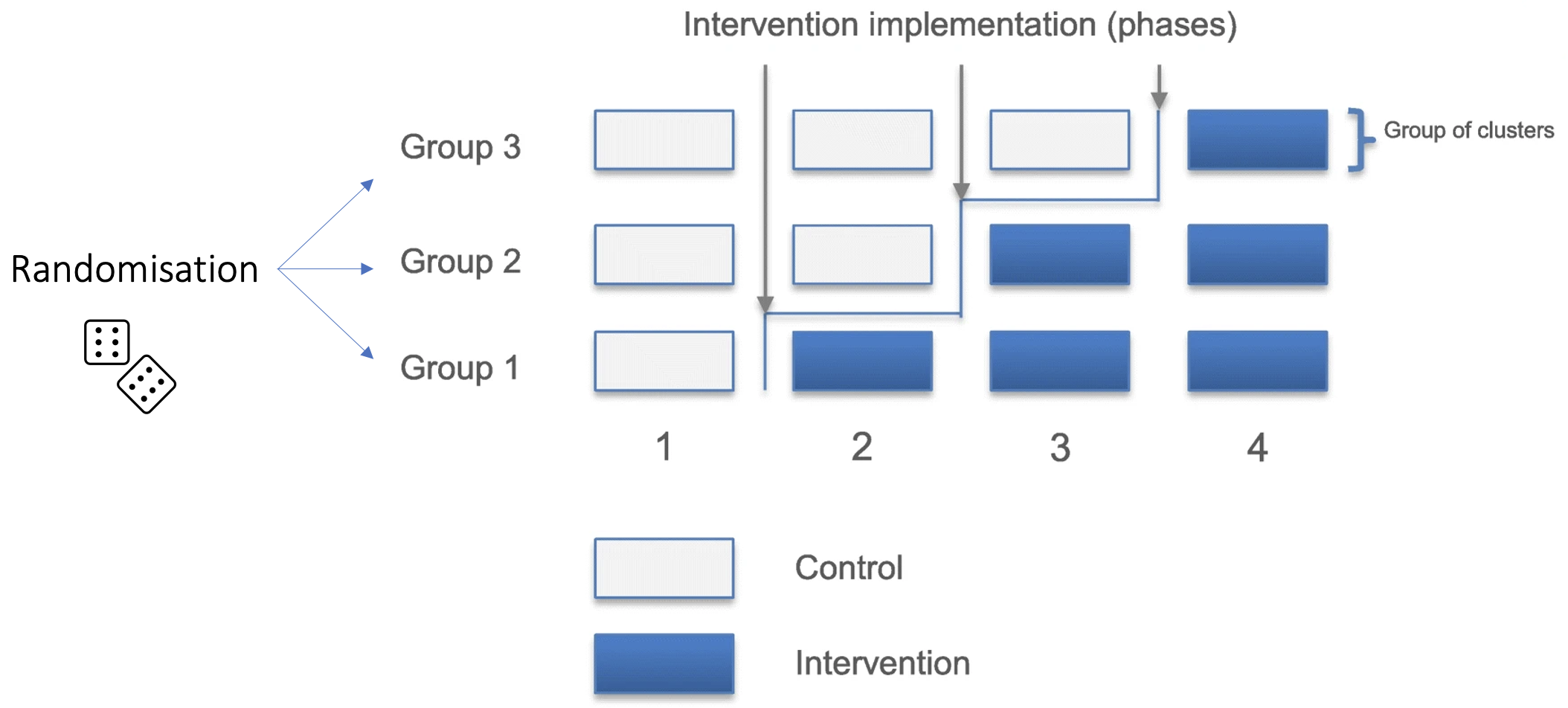
Stepped wedge cluster randomized trial: each sites starts with standard of care, then switches to intervention at after a certain number of periods (1-3), as allocated by site-level randomisation.
Period: 3-4 months, or until a certain (variable) number of patients were enrolled
Comments:
- highly variable period lengths between clusters
- COVID pandemic fell in middle, with stopped recruitement in some countries -> later phases were extended
- Advantages: allows for fast intervention (no need to wait for patient to be randomized), and testing implementation of protocol
Intervention
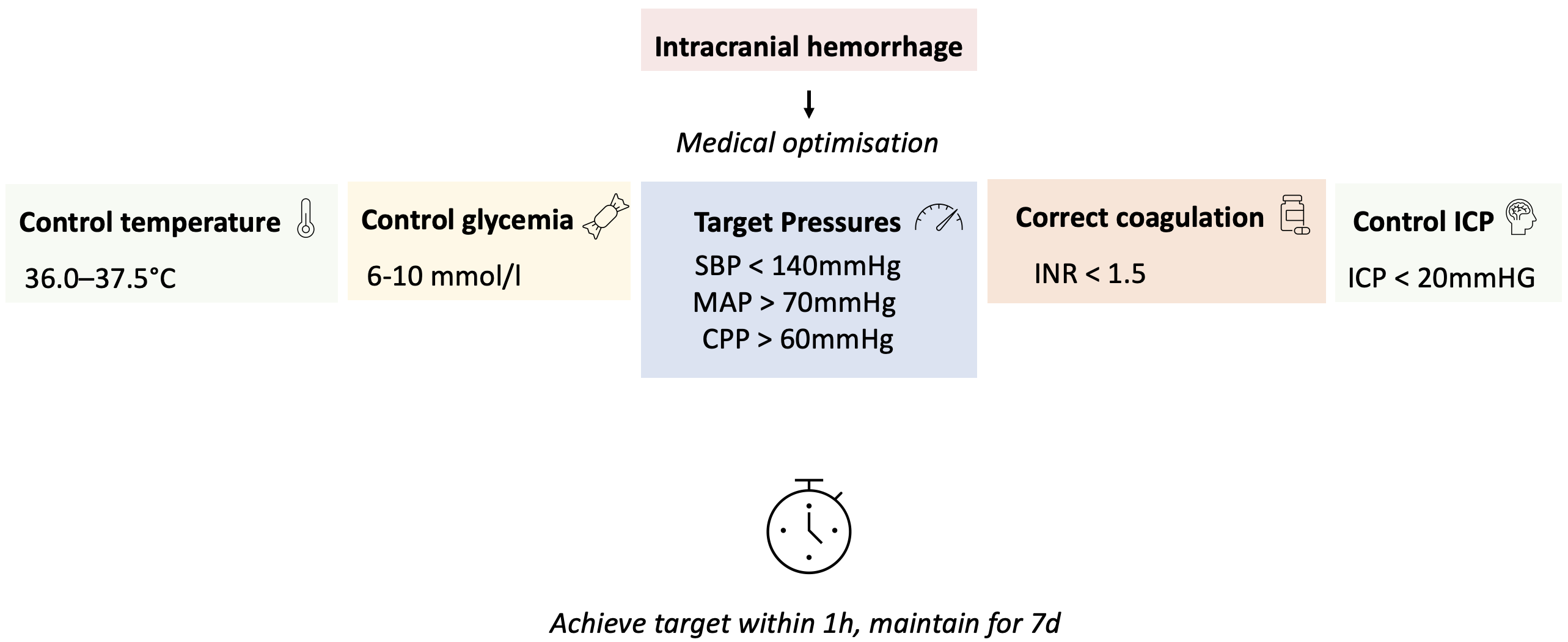
Bundle
- Target SBP 130-140mmHg
- Target INR < 1.5
- Glucose control
- Non-diabetic: 6.1-7.8 mM
- Diabetic: 7.8-10.0 mM
- Comment: very tight range, lower than NICE-SUGAR (<10) & Quality in acute stroke care (< 11), higher than Leuven trials (4.4-6.1)
- Temperature control: < 37.5°C
Targets were to be achieved within 1h, and maintained for 7d.
Protocol implementation
- Before intervention: 7-10d online training
- During intervention:
- Center specific protocol (targeted to available drugs)
- Monitoring of SBP / INR / glycemia / temperature (trial staff)
- Monthly feedback
- 2 QI Meetings
Outcomes
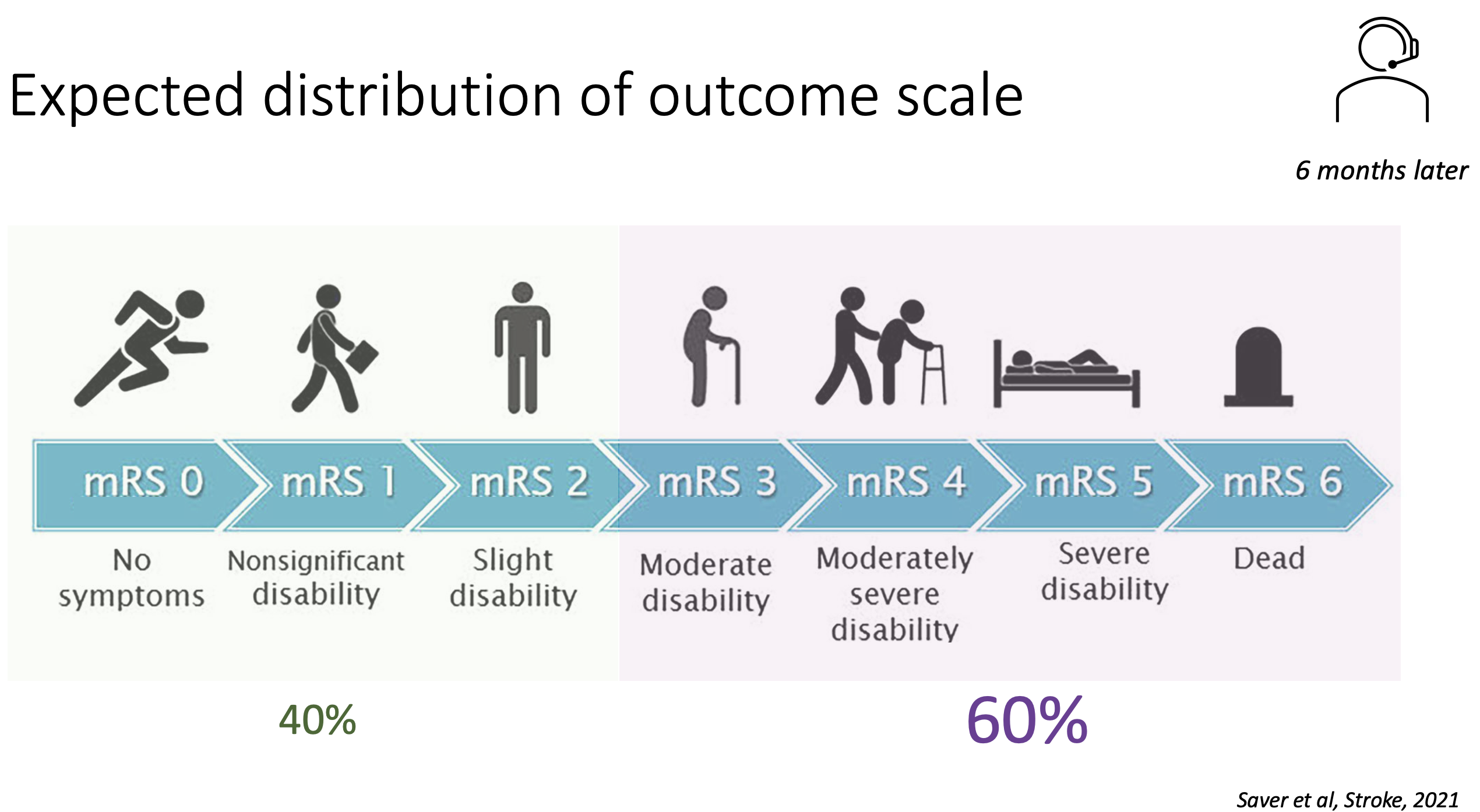
Primary outcome: ordinal shift on modified Rankin Scale (mRS) at 6 months
Main secondary outcomes: mRS ≤ 2 vs ≥ 3, mortality, quality of life, residence
Safety Outcomes: serious adverse events (self reported, only if life-threatening / disability / or triggering readmission / prolongation)
Assessment: by phone by “blinded” personnel (central office only in China / Chile / Brazil / Nigeria) - otherwise located at hospitals (who probably knew the current protocol being applied)
Results
Trial conduct
Recruitment: 121 sites, 7036 patients randomized
- 30% patients excluded upon screening, but adequate reasons (mostly presentation > 6h, no consent, structural cause)
- Minimum 8% lost to follow-up & 11% with no primary outcome data
- –> Slightly underpowered (planned for 90% power: 110sites, 8360 patients)
Population: Randomisation worked, well-balanced between groups
- at baseline: compared to Swiss ICH patients, these patients tended to be ~10y younger (62y), fitter (77% pre-mRS 0), and with less AF / D2 / coronary disease, but similar level of hypertension
- at presentation: TAS 175 mmHg, moderately severe neuro deficit (NIHSS 13), GCS 12
- Only very few with altered T° / INR (<2%)
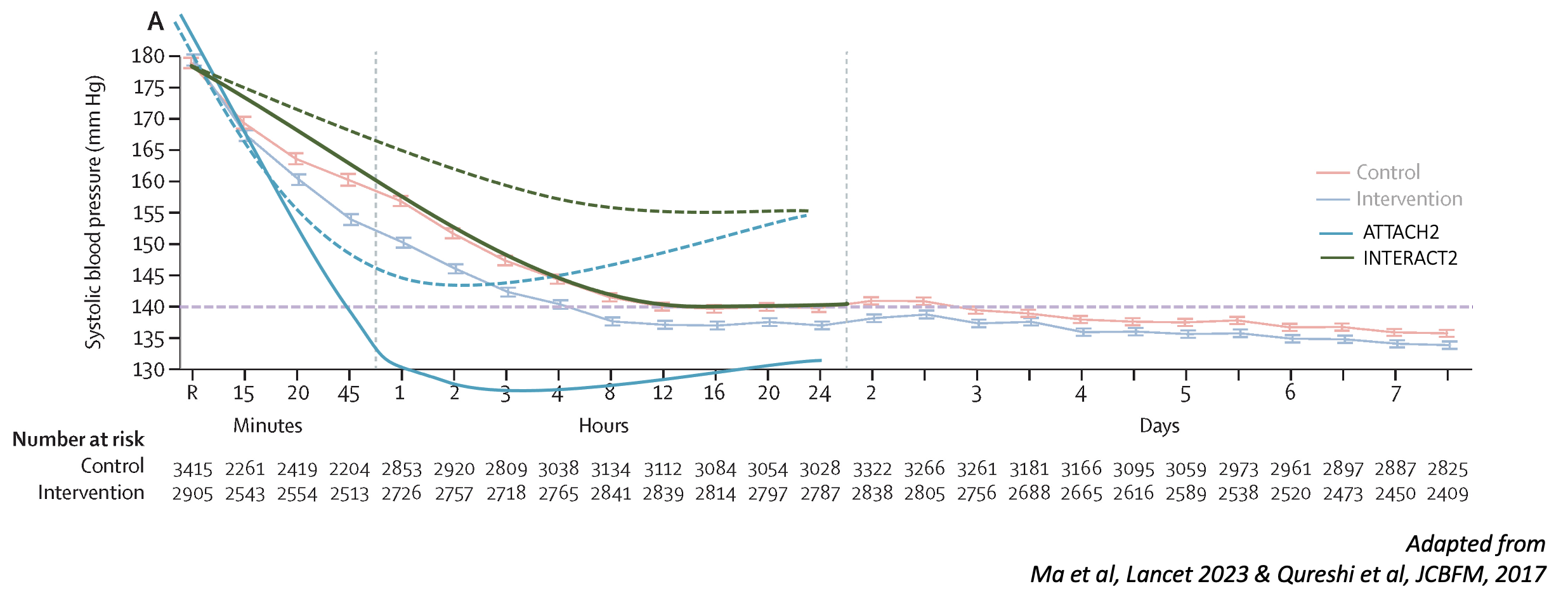
Intervention (BP): Small but significant difference in SBP between groups
- on average: target achieved at 4h vs 8h
- at 1h: 150 vs 160mmHg
- Main agents used
- IV: urapidil (60%), nicardipine (8.5%), sodium nitroprusside, labetalol, nimodipine, very little clevidipine (~2%)
- PO: ACEi (~60%), CCB (~76%)
- Compared to prior trials:
- Less intensive than ATACH2 (which had shown an increase in AKI)
- More than INTERACT2
Intervention (rest of bundle): no significant separation between groups
Outcomes
Primary outcome: favorable shift in mRS in intervention group (OR 0.86; 95% CI 0.76-0.97, p=0.015), consistent across all adjustments/calculations
- Statistical calculation of primary outcome had to be changed post-protocol. Initially it was planned to adjust for time passing by correcting for (= using as fixed factor) period, however as the period length varied a lot, this resulted in a non-significant primary outcome (probably because of correlation between intervention assignment and period). The authors changed this to correcting for time by using calendar time periods. To a statistical novice, this seems to be okay.
- Size in shift in primary outcome not very impressive, with notably less mRs 0 & more mRs 3?
Secondary outcomes: all strictly non-significant, but trends consistent with primary outcome
- notably adjusted risk of death 11% vs 14% (not significant)
Subgroups: trends consistent across subgroups, no clear effect modifiers
Safety outcomes: reported reduced adverse events
- gross prevalence seems under-reported (ex pneumonia prevalence after ICH should be 10-60%)
- a reduction in probably unrelated adverse events is reported (e.g. gastro-intestinal disorders)
- no diff. in AKI, but probably underreported as this was not systematically assessed
Other: no difference in hematoma expansion
Assessment
Weaknesses
- Small effect in primary outcome
- Generalizability? Mostly China & LMICs, patients with no anti-coagulation
- Not fully blinded (outcome assessment was probably not blinded in some sites)
- Outcome assessment by phone
- Bundle: Although BP was most prominent aspect, effect of other interventions cannot be excluded
- Hawthorne effect: sites were aware of being in a trial, and were probably more attentive to patient care when in the bundle phase
- Renal side effects not systematically assessed
Strengths
- Large international, well conducted RCT
- Pragmatic bundle, large scale implementation
- Relevant global health issue
Interpretation
- Bayesian: given a probable a priori beneficial effect of BP lowering, this trial provides further evidence for a beneficial effect of intensive BP lowering in ICH
- Probably an absolute small effect (~-2-3% mortality), but applied on large scale
- Implementation of a bundle/guideline should be recommended, as it carries further benefits
- organising care & promoting monitoring
- defining goals & interventions renders a disease without hope more manageable, and thus probably improves care
- highlights urgency of hemorrhagic stroke - time is brain
- Exact mechanism of benefit of BP lowering is still unclear (no diff. in hematoma expansion)
Bottomline: Bundle of intensive BP lowering (target 130-140mmHg), glucose & temperature control, and reversal of anticoagulation in ICH patients improves outcomes.
Adapted algorithm for ICH management
References:
Greenberg, S. M. et al. 2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 53, e282–e361 (2022).
Qureshi, A. I. Acute Hypertensive Response in Patients With Stroke. Circulation 118, 176–187 (2008).
Parry-Jones, A. R. et al. An Intracerebral Hemorrhage Care Bundle Is Associated with Lower Case Fatality. Ann Neurol 86, 495–503 (2019).
Investigators, A. T. of A. C. H. (ATACH). Antihypertensive treatment of acute cerebral hemorrhage*. Critical Care Medicine 38, 637 (2010).
Moullaali, T. J. et al. Blood pressure control and clinical outcomes in acute intracerebral haemorrhage: a preplanned pooled analysis of individual participant data. The Lancet Neurology 18, 857–864 (2019).
Yu, K., Sun, Y., Guo, K., Peng, J. & Jiang, Y. Early blood pressure management in hemorrhagic stroke: a meta-analysis. J Neurol 270, 3369–3376 (2023).
Moullaali, T. J. et al. Early lowering of blood pressure after acute intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. J Neurol Neurosurg Psychiatry 93, 6–13 (2022).
Anderson, C. S., Selim, M. H., Molina, C. A. & Qureshi, A. I. Intensive Blood Pressure Lowering in Intracerebral Hemorrhage. Stroke 48, 2034–2037 (2017).
Anderson, C. S. et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol 7, 391–399 (2008).
Qureshi, A. I. et al. Intensive Blood-Pressure Lowering in Patients with Acute Cerebral Hemorrhage. New England Journal of Medicine 375, 1033–1043 (2016).
Song, L. et al. INTEnsive care bundle with blood pressure reduction in acute cerebral hemorrhage trial (INTERACT3): study protocol for a pragmatic stepped-wedge cluster-randomized controlled trial. Trials 22, 943 (2021).
Qureshi, A. I., Mendelow, A. D. & Hanley, D. F. Intracerebral haemorrhage. The Lancet 373, 1632–1644 (2009).
Anderson, C. S. et al. Rapid Blood-Pressure Lowering in Patients with Acute Intracerebral Hemorrhage. New England Journal of Medicine 368, 2355–2365 (2013).
Burns, J., Fisher, J. & Cervantes-Arslanian, A. Recent Advances in the Acute Management of Intracerebral Hemorrhage. Neurosurgery Clinics of North America 29, 263–272 (2018).
Ma, L. et al. The third Intensive Care Bundle with Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT3): an international, stepped wedge cluster randomised controlled trial. The Lancet 402, 27–40 (2023).
Lectures: „Evidence based management of intracerebral hemorrhage - An update”, AI Quereshi, 2022
FOAM: INTERACT3, The sceptics guide to emergency medecinte. Pallaci & Hunter.