An Approach to Diffusion Tensor Imaging of Auditory Brainstem Implant Candidates
Published:
Context
What is an ABI?
The auditory brainstem implant (ABI) provides sound detection for patients who are not candidates for a cochlear implant due to anatomic constraints. The ABI bypasses the cochlea and cochlear nerve to electrically stimulate second order auditory neurons of the cochlear nucleus (found in the pontomedullary junction of the brainstem).
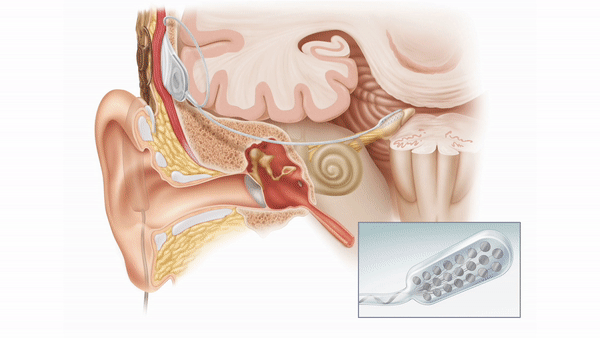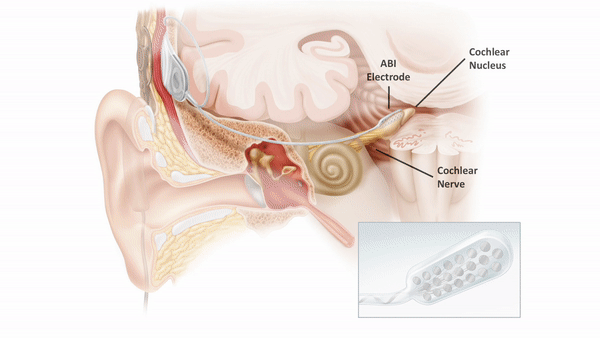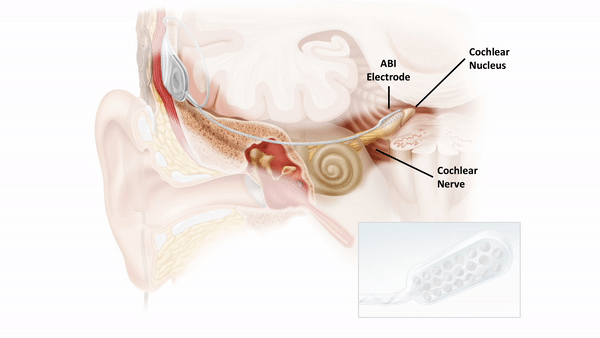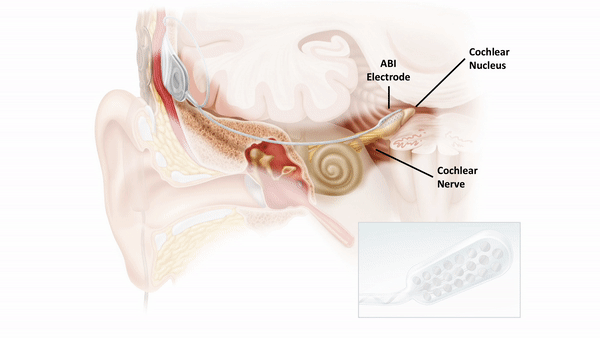
Unfortunately, hearing outcomes among ABI recipients remain highly variable and performance lags significantly behind cochlear implant users. Anatomic differences, including size of the residual cochlear nerve or presence of a cerebellopontine angle tumor, as well as differences in electrode position may influence audiometric outcomes.
Prior research also suggests that white matter organization, when visualized with diffusion tensor imaging, correlates with auditory performance in patients with cochlear implants. Diffusion tensor imaging (DTI) provides a specific type of modeling data to reveal structural integrity of white matter in vivo by detecting the quantity and directionality of water diffusion. Quantitative analysis of white matter microstructure using DTI may enable greater insight into ABI outcomes perioperatively.
However, ABI candidates, especially those affected by Neurofibromatosis type 2 (NF2), represent a particular challenge for the visualization of white matter tracts due to deformities in the posterior fossa from large tumor load and hardware such as auditory implants and cranioplasty material.
Aim
The aim of this study was to examine the feasibility of utilizing DTI to assess white matter microstructures along the auditory pathways in ABI candidates.
Methods
A total of eight patients diagnosed with NF2 were implanted and scanned perioperatively. The diffusion weighed sequences were imported and registered to standard space. After removing all non-brain tissue, diffusion tensors were estimated, and FA and MD values were extracted on selected regions of interest (ROIs).
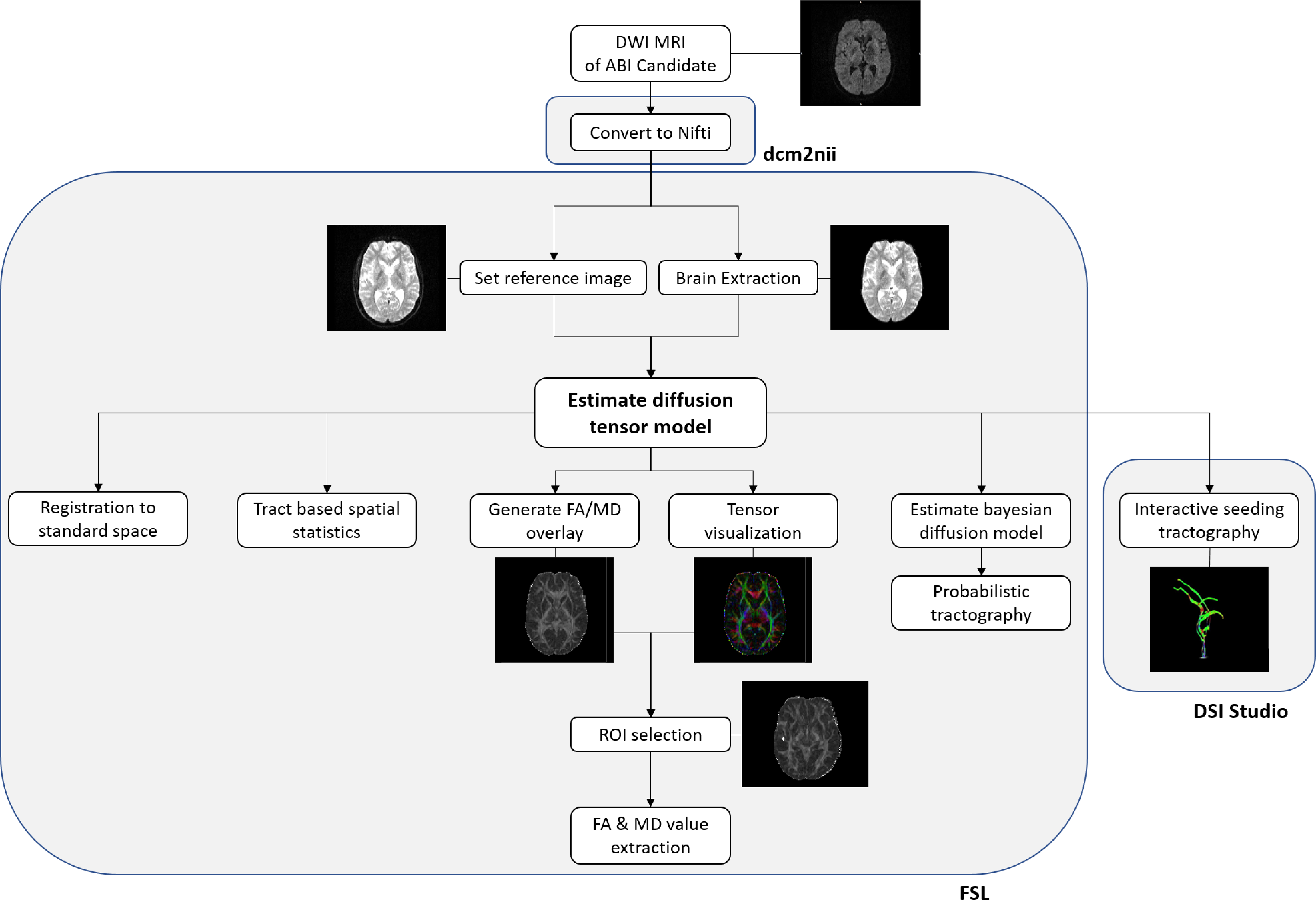
Results
- ROIs were extracted and DTI indices could be measured in 76% of subjects
- Fiber tracking could be performed
- Limitations were identified
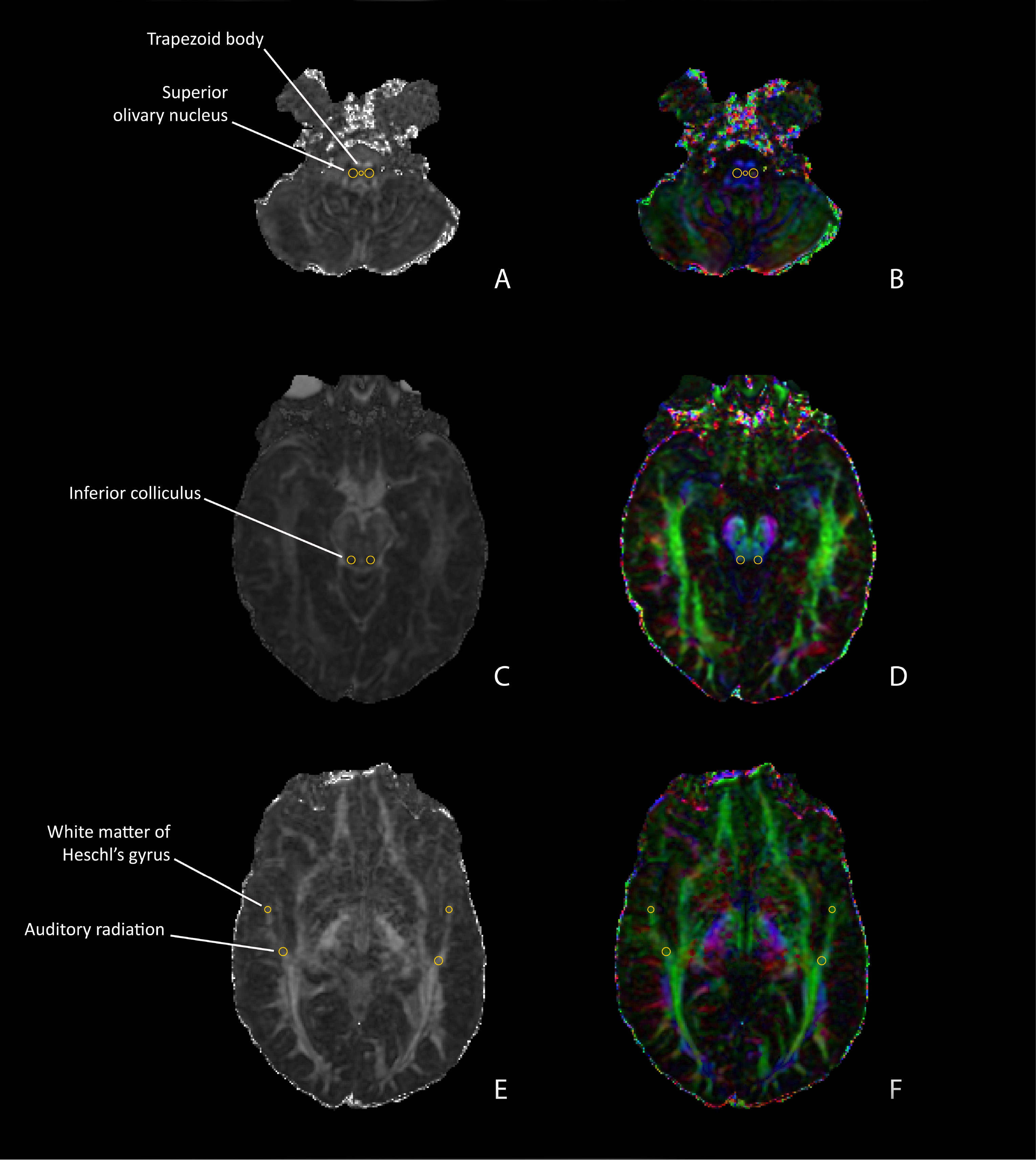 Representative DTI scans (left: FA/MD overlap map; right: FA-modulated vector map) of selected ROIs. A. Trapezoid body and superior olivary nuclei. B. Inferior colliculi. C. Auditory radiations and white matter of Heschl’s gyrus.
Representative DTI scans (left: FA/MD overlap map; right: FA-modulated vector map) of selected ROIs. A. Trapezoid body and superior olivary nuclei. B. Inferior colliculi. C. Auditory radiations and white matter of Heschl’s gyrus.
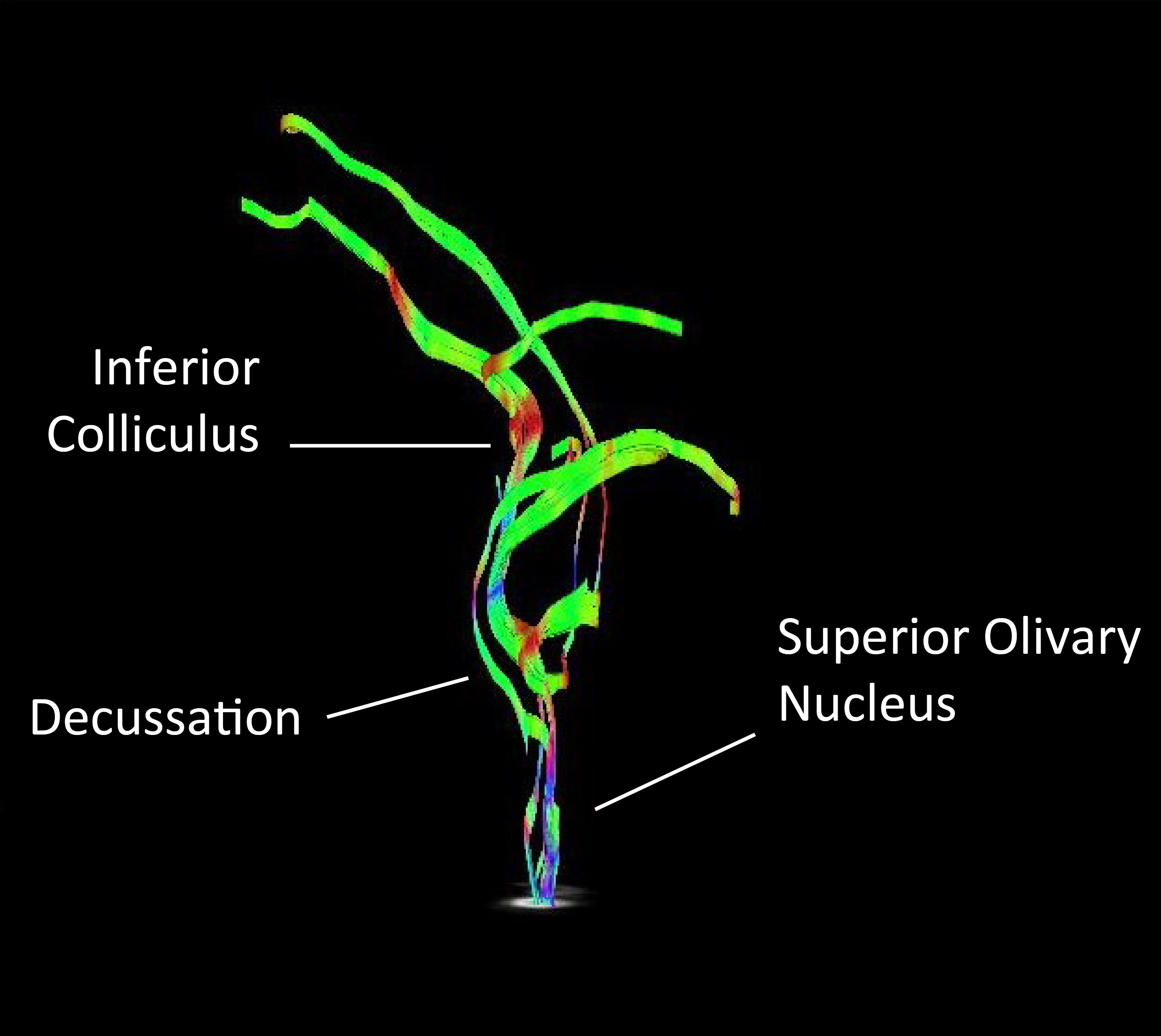 Representative tractography of fibers originating in the superior olivary nucleus (coronal view). Tracts decussating before reaching the contralateral inferior colliculus can be visualized.
Representative tractography of fibers originating in the superior olivary nucleus (coronal view). Tracts decussating before reaching the contralateral inferior colliculus can be visualized.
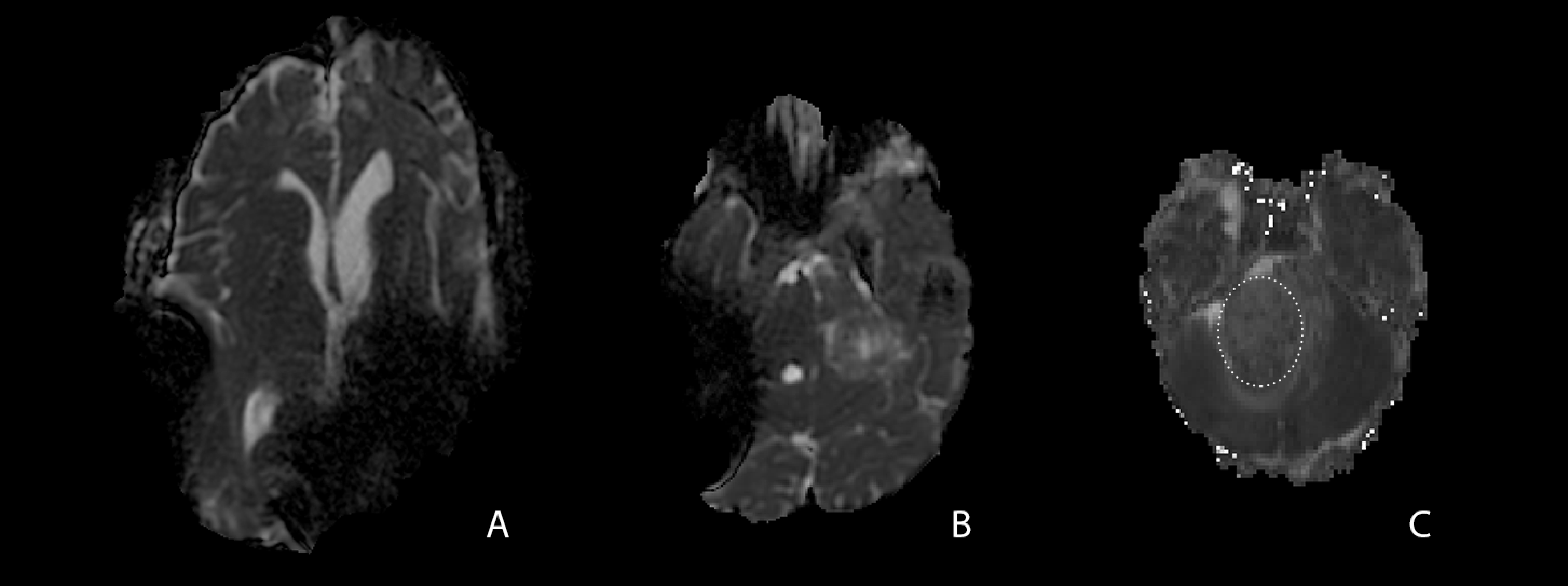 Representative FA/MD overlay maps for ROIs with missing values (axial view). A. Susceptibility artifact due to left cochlear implant. B. Susceptibility artifact due to right ABI. C. Tumor (dotted line) compressing brainstem.
Representative FA/MD overlay maps for ROIs with missing values (axial view). A. Susceptibility artifact due to left cochlear implant. B. Susceptibility artifact due to right ABI. C. Tumor (dotted line) compressing brainstem.
Discussion
At the time, this was the first study to describe the use of DTI in candidates for the ABI in a clinical setting, allowing the measure of FA and MD at specific ROIs along the auditory pathway. This matters, as earlier studies by Huang and colleagues have shown that FA values of the same ROIs correlate with auditory outcome after cochlear implantation. Combined with the approach described here, this suggests the possibility for a tool to predict ABI outcomes preoperatively. Moreover, Scholz et al. describe the effect of motor training on FA measures on selected ROIs. Applying this methodology to ABI users would allow to gain great insight to the physiological changes following implantation and might hint to further possibilities to enhance postoperative adaptation.
Limitations
- Small sample size
- Inconsistent DTI availability
- Low DTI resolution
Conclusions
| Technique | Utility | Feasibility in ABI patients |
|---|---|---|
| FA/MD measures | Quantitative analysis (ROIs) | Yes |
| FA-modulated vector map | Visualization of fiber direction | Yes |
| Whole Brain Image Registration | Comparative analysis | No |
| Tract-Based Spatial Statistics | Quantitative analysis (whole brain) | No |
| Tractography | Qualitative analysis & Tract visualization | Yes |
Feasibility of diffusion tensor imaging techniques and measures in ABI candidates
Personal Context
I worked on this project during my time at the Lee/Brown lab, Harvard/MEEI, with the continuous support of V. Kanumuri, MD. In the end, I never had the occasion to fully complete and publish it.
Code availability
All code is available at my github. It is mostly based on FSL.
If you are interested in reading the original paper, don’t hesitate to contact me.
Video Summary
References
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208-219.
- Huang L, Zheng W, Wu C, Wei X, Wu X, Wang Y, et al. Diffusion Tensor Imaging of the Auditory Neural Pathway for Clinical Outcome of Cochlear Implantation in Pediatric Congenital Sensorineural Hearing Loss Patients. PLoS ONE. 2015;10(10):e0140643.
- Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009 Nov;12(11):1370–1.
- Potthoff, M. (2013). ABI how it works image [image format]. retrieved from http://newsroom.hei.org/news/fda-approves-clinical-trial-of-242830


